Live Clean Baby Gentle Moisture Diaper Rash Cream -- 2.6 Oz Reviews
© Prototype past Gerd Altmann from Pixabay
According to a study that examined how informed consent is given to COVID-19 vaccine trial participants, disclosure forms fail to inform volunteers that the vaccine might make them susceptible to more astringent disease if they're exposed to the virus.
The study,ane "Informed Consent Disclosure to Vaccine Trial Subjects of Chance of COVID-nineteen Vaccine Worsening Clinical Illness," published in the International Journal of Clinical Exercise, October 28, 2020, points out that "COVID-19 vaccines designed to elicit neutralizing antibodies may sensitize vaccine recipients to more severe affliction than if they were not vaccinated."
"Vaccines for SARS, MERS and RSV take never been canonical, and the data generated in the evolution and testing of these vaccines suggest a serious mechanistic concern: that vaccines designed empirically using the traditional approach (consisting of the unmodified or minimally modified coronavirus viral fasten to arm-twist neutralizing antibodies), be they equanimous of poly peptide, viral vector, Dna or RNA and irrespective of delivery method, may worsen COVID-19 disease via antibiotic-dependent enhancement (ADE)," the newspaper states.
"This risk is sufficiently obscured in clinical trial protocols and consent forms for ongoing COVID-xix vaccine trials that adequate patient comprehension of this risk is unlikely to occur, obviating truly informed consent by subjects in these trials.
The specific and significant COVID-19 risk of ADE should accept been and should be prominently and independently disclosed to research subjects currently in vaccine trials, as well as those beingness recruited for the trials and future patients after vaccine approval, in guild to meet the medical ethics standard of patient comprehension for informed consent."
What Is Antibody-Dependent Enhancement?
As noted by the authors of that International Journal of Clinical Practice paper, previous coronavirus vaccine efforts — for severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and respiratory syncytial virus (RSV) — have revealed a serious concern: The vaccines have a trend to trigger antibiotic-dependent enhancement.
What exactly does that hateful? In a nutshell, it means that rather than enhance your immunity against the infection, the vaccine actually enhances the virus' ability to enter and infect your cells, resulting in more than severe illness than had you lot non been vaccinated. two
This is the exact reverse of what a vaccine is supposed to practise, and a significant problem that has been pointed out from the very beginning of this push for a COVID-19 vaccine. The 2003 review paper "Antibody-Dependent Enhancement of Virus Infection and Illness" explains it this way:3
"In general, virus-specific antibodies are considered antiviral and play an of import part in the control of virus infections in a number of ways. Nonetheless, in some instances, the presence of specific antibodies tin be beneficial to the virus. This activeness is known equally antibody-dependent enhancement (ADE) of virus infection.
The ADE of virus infection is a phenomenon in which virus-specific antibodies enhance the entry of virus, and in some cases the replication of virus, into monocytes/macrophages and granulocytic cells through interaction with Fc and/or complement receptors.
This phenomenon has been reported in vitro and in vivo for viruses representing numerous families and genera of public health and veterinarian importance. These viruses share some common features such every bit preferential replication in macrophages, ability to plant persistence, and antigenic diversity. For some viruses, ADE of infection has become a great concern to disease command by vaccination."
Previous Coronavirus Vaccine Efforts Accept All Failed
In my May 2020 interview above with Robert Kennedy Jr., he summarized the history of coronavirus vaccine evolution, which began in 2002, post-obit three consecutive SARS outbreaks. By 2012, Chinese, American and European scientists were working on SARS vaccine evolution, and had virtually thirty promising candidates.
Of those, the 4 best vaccine candidates were and then given to ferrets, which are the closest analogue to human being lung infections. In the video beneath, which is a select outtake from my total interview, Kennedy explains what happened next. While the ferrets displayed robust antibody response, which is the metric used for vaccine licensing, in one case they were challenged with the wild virus, they all became severely ill and died.
The same affair happened when they tried to develop an RSV vaccine in the 1960s. RSV is an upper respiratory illness that is very like to that acquired past coronaviruses. At that time, they had decided to skip animal trials and go directly to human trials.
"They tested it on I call up nigh 35 children, and the aforementioned thing happened," Kennedy said. "The children developed a champion antibody response — robust, durable. It looked perfect [but when] the children were exposed to the wild virus, they all became sick. Two of them died. They abased the vaccine. It was a big embarrassment to FDA and NIH."
Neutralizing Versus Binding Antibodies
Coronaviruses produce non just one but two different types of antibodies:
- Neutralizing antibodies,4 also referred to as immunoglobulin G (IgG) antibodies, that fight the infection
- Binding antibodies5 (also known as non-neutralizing antibodies) that cannot preclude viral infection
Instead of preventing viral infection, binding antibodies trigger an aberrant immune response known as "paradoxical immune enhancement." Another way to await at this is your immune system is actually backfiring and not operation to protect you but actually making you lot worse.
Many of the COVID-19 vaccines currently in the running are using mRNA to instruct your cells to make the SARS-CoV-2 spike protein (Southward protein). The spike protein, which is what attaches to the ACE2 receptor of the prison cell, is the offset phase of the two-stage procedure viruses use to proceeds entry into cells.
The idea is that past creating the SARS-CoV-2 spike poly peptide, your immune system will commence production of antibodies, without making you ill in the process. The key question is, which of the ii types of antibodies are being produced through this process?
Without Neutralizing Antibodies, Look More Severe Affliction
In an Apr 2020 Twitter thread,6 The Immunologist noted: "While developing vaccines ... and because immunity passports, we must kickoff understand the complex function of antibodies in SARS, MERS and COVID-xix." He goes on to list several coronavirus vaccine studies that have raised concerns about ADE.
The beginning is a 2017 written report7 in PLOS Pathogens, "Enhanced Inflammation in New Zealand White Rabbits When MERS-CoV Reinfection Occurs in the Absence of Neutralizing Antibiotic," which investigated whether getting infected with MERS would protect the subject against reinfection, as is typically the case with many viral illnesses. (Pregnant, in one case you recover from a viral infection, say measles, you're immune and won't contract the affliction again.)
To determine how MERS affects the allowed system, the researchers infected white rabbits with the virus. The rabbits got sick and developed antibodies, but those antibodies were not the neutralizing kind, meaning the kind of antibodies that block infection. Equally a result, they were not protected from reinfection, and when exposed to MERS for a second time, they became ill over again, and more than severely then.
"In fact, reinfection resulted in enhanced pulmonary inflammation, without an associated increase in viral RNA titers," the authors noted. Interestingly, neutralizing antibodies were elicited during this second infection, preventing the animals from being infected a 3rd time. According to the authors:
"Our data from the rabbit model suggests that people exposed to MERS-CoV who neglect to develop a neutralizing antibody response, or persons whose neutralizing antibody titers have waned, may exist at risk for severe lung affliction on re-exposure to MERS-CoV."
In other words, if the vaccine does non result in a robust response in neutralizing antibodies, you might be at adventure for more severe lung illness if you're infected with the virus.
And here's an important point: COVID-nineteen vaccines are NOT designed to foreclose infection. Equally detailed in "How COVID-xix Vaccine Trials Are Rigged," a "successful" vaccine only needs to reduce the severity of the symptoms. They're not even looking at reducing infection, hospitalization or death rates.
ADE in Dengue Infections
The Dengue virus is also known to crusade ADE. Every bit explained in a Swiss Medical Weekly paper published in Apr 2020:8
"The pathogenesis of COVID-19 is currently believed to proceed via both straight cytotoxic and immune-mediated mechanisms. An additional mechanism facilitating viral cell entry and subsequent harm may involve the and so-called antibiotic-dependent enhancement (ADE).
ADE is a very well-known cascade of events whereby viruses may infect susceptible cells via interaction between virions complexed with antibodies or complement components and, respectively, Fc or complement receptors, leading to the distension of their replication.
This phenomenon is of enormous relevance not merely for the understanding of viral pathogenesis, but also for developing antiviral strategies, notably vaccines ...
There are 4 serotypes of Dengue virus, all eliciting protective immunity. However, although homotypic protection is long-lasting, cross-neutralizing antibodies against different serotypes are short-lived and may terminal but upwardly to 2 years.
In Dengue fever, reinfection with a different serotype runs a more than severe course when the protective antibody titer wanes. Hither, non-neutralizing antibodies take over neutralizing ones, demark to Dengue virions, and these complexes mediate the infection of phagocytic cells via interaction with the Fc receptor, in a typical ADE.
In other words, heterotypic antibodies at subneutralizing titres business relationship for ADE in persons infected with a serotype of Dengue virus that is different from the offset infection.
Cantankerous-reactive neutralizing antibodies are associated with decreased odds of symptomatic secondary infection, and the higher the titer of such antibodies post-obit the primary infection, the longer the filibuster to symptomatic secondary infection ..."
The paper goes on to item results from follow-up investigations into the Dengue vaccine, which revealed the hospitalization charge per unit for Dengue amidst vaccinated children under the age of 9 was greater than the rate amidst controls. The caption for this appears to be that the vaccine mimicked a primary infection, and as that immunity waned, the children became susceptible to ADE when they encountered the virus a 2d time. The writer explains:
"A postal service hoc analysis of efficacy trials, using an anti-nonstructural poly peptide 1 immunoglobulin Thou (IgG) enzyme-linked immunosorbent assay (ELISA) to distinguish antibodies elicited by wild-blazon infection from those post-obit vaccination, showed that the vaccine was able to protect confronting severe Dengue [in] those who had been exposed to the natural infection before vaccination, and that the hazard of severe clinical upshot was increased amid seronegative persons.
Based on this, a Strategic Counselor Group of Experts convened by World Health System (WHO) ended that only Dengue seropositive persons should exist vaccinated whenever Dengue control programs are planned that include vaccination."
ADE in Coronavirus Infections
This could terminate upwards being of import for the COVID-19 vaccine. Hypothetically speaking, if SARS-CoV-2 works similar Dengue, which is also caused past an RNA virus, then anyone who has not tested positive for SARS-CoV-2 might really be at increased risk for astringent COVID-19 afterwards vaccination, and only those who have already recovered from a bout of COVID-19 would exist protected confronting severe affliction past the vaccine.
To be articulate, nosotros do not know whether that is the example or not, merely these are important areas of inquiry and the current vaccine trials will simply not be able to answer this important question.
The Swiss Medical Weekly newspaper 9 also reviews the bear witness of ADE in coronavirus infections, citing research showing inoculating cats against the feline infectious peritonitis virus (FIPV) — a feline coronavirus — increases the severity of the disease when challenged with the same FIPV serotype equally that in the vaccine.
Experiments have shown immunization with a variety of SARS vaccines resulted in pulmonary immunopathology once challenged with the SARS virus.
The paper also cites research showing "Antibodies elicited past a SARS-CoV vaccine enhanced infection of B jail cell lines in spite of protective responses in the hamster model." Another newspaper,10 "Antibody-Dependent SARS Coronavirus Infection Is Mediated by Antibodies Against Spike Proteins," published in 2014, institute that:
"... higher concentrations of anti-sera confronting SARS-CoV neutralized SARS-CoV infection, while highly diluted anti-sera significantly increased SARS-CoV infection and induced college levels of apoptosis.Results from infectivity assays betoken that SARS-CoV ADE is primarily mediated by diluted antibodies against envelope spike proteins rather than nucleocapsid proteins. We also generated monoclonal antibodies against SARS-CoV spike proteins and observed that nigh of them promoted SARS-CoV infection.
Combined, our results suggest that antibodies against SARS-CoV spike proteins may trigger ADE effects. The data raise new questions regarding a potential SARS-CoV vaccine ..."
A study11 that ties into this was published in the journal JCI Insight in 2019. Here, macaques vaccinated with a modified vaccinia Ankara (MVA) virus encoding total-length SARS-CoV spike protein ended upward with more severe lung pathology when the animals were exposed to the SARS virus. And, when they transferred anti-spike IgG antibodies into unvaccinated macaques, they developed astute diffuse alveolar impairment, likely past "skewing the inflammation-resolving response."
SARS Vaccine Worsens Infection Afterwards Challenge With SARS-CoV
An interesting 2012 paper 12 with the telling title, "Immunization with SARS Coronavirus Vaccines Leads to Pulmonary Immunopathology on Claiming with the SARS Virus," demonstrates what many researchers at present fear, namely that COVID-xix vaccines may end upwardly making people more decumbent to severe SARS-CoV-two infection.
The paper reviews experiments showing immunization with a variety of SARS vaccines resulted in pulmonary immunopathology one time challenged with the SARS virus. As noted past the authors: xiii
Inactivated whole virus vaccines whether inactivated with formalin or beta propiolactone and whether given with our without alum adjuvant exhibited a Th2-type immunopathologic in lungs later on claiming.Every bit indicated, two reports attributed the immunopathology to presence of the Due north protein in the vaccine; however, nosotros found the same immunopathologic reaction in animals given S protein vaccine only, although it appeared to be of lesser intensity.
Thus, a Th2-type immunopathologic reaction on claiming of vaccinated animals has occurred in 3 of iv animal models (non in hamsters) including ii different inbred mouse strains with four different types of SARS-CoV vaccines with and without alum adjuvant. An inactivated vaccine grooming that does not induce this result in mice, ferrets and nonhuman primates has non been reported.
This combined experience provides concern for trials with SARS-CoV vaccines in humans. Clinical trials with SARS coronavirus vaccines have been conducted and reported to induce antibody responses and to be 'condom.' However, the evidence for safe is for a short menstruation of observation.
The business organization arising from the nowadays report is for an immunopathologic reaction occurring amidst vaccinated individuals on exposure to infectious SARS-CoV, the basis for developing a vaccine for SARS. Additional safety concerns relate to effectiveness and rubber confronting antigenic variants of SARS-CoV and for safety of vaccinated persons exposed to other coronaviruses, especially those of the type 2 group."
The Elderly Are Most Vulnerable to ADE
On meridian of all of these concerns, there'south show showing the elderly — who are virtually vulnerable to severe COVID-19 — are as well the near vulnerable to ADE. Preliminary research findings14 posted on the preprint server medRxiv at the end of March 2020 reported that middle-aged and elderly COVID-xix patients have far college levels of anti-spike antibodies — which, once more, increment infectivity — than younger patients.
Allowed Enhancement Is a Serious Concern
Another newspaper worth mentioning is the May 2020 mini review15 "Affect of Allowed Enhancement on COVID-19 Polyclonal Hyperimmune Globulin Therapy and Vaccine Evolution." As in many other papers, the authors indicate out that:sixteen
"While evolution of both hyperimmune globulin therapy and vaccine against SARS-CoV-2 are promising, they both pose a common theoretical safety concern. Experimental studies have suggested the possibility of immune-enhanced disease of SARS-CoV and MERS-CoV infections, which may thus similarly occur with SARS-CoV-two infection ...
Immune enhancement of illness can theoretically occur in 2 means. Firstly, not-neutralizing or sub-neutralizing levels of antibodies can enhance SARS-CoV-ii infection into target cells.
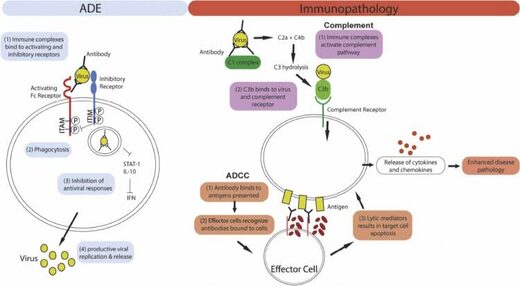
Secondly, antibodies could enhance inflammation and hence severity of pulmonary affliction. An overview of these antibody dependent infection and immunopathology enhancement effects are summarized in Fig. 1 ...
Currently, there are multiple SARS-CoV and MERS-CoV vaccine candidates in pre-clinical or early stage clinical trials. Beast studies on these CoVs have shown that the fasten (S) poly peptide-based vaccines (specifically the receptor binding domain, RBD) are highly immunogenic and protective against wild-type CoV claiming.
Vaccines that target other parts of the virus, such as the nucleocapsid, without the S poly peptide, have shown no protection against CoV infection and increased lung pathology. Yet, immunization with some S poly peptide based CoV vaccines have also displayed signs of enhanced lung pathology following challenge.
Hence, as well the choice of antigen target, vaccine efficacy and risk of immunopathology may be dependent on other ancillary factors, including adjuvant conception, age at vaccination ... and route of immunization."
© manufactures.mercola.com
Figure one: Mechanism of ADE and antibiotic mediated immunopathology. Left panel: For ADE, allowed complex internalization is mediated past the engagement of activating Fc receptors on the jail cell surface. Co-ligation of inhibitory receptors then results in the inhibition of antiviral responses which leads to increased viral replication. Correct panel: Antibodies can cause immunopathology by activating the complement pathway or antibody-dependent cellular cytotoxicity (ADCC). For both pathways, excessive allowed activation results in the release of cytokines and chemokines, leading to enhanced illness pathology.
Do a Risk-Do good Assay Before Making Upwardly Your Mind
In all likelihood, regardless of how constructive (or ineffective) the COVID-19 vaccines end upward being, they'll be released to the public in relatively short order. Most predict one or more vaccines will be ready sometime in 2021.
Ironically, the information 17,18,19 nosotros now have no longer support a mass vaccination mandate, considering the lethality of COVID-19 is lower than the influenza for those under the age of threescore. twenty If you're under the historic period of forty, your take chances of dying from COVID-19 is just 0.01%, meaning you take a 99.99% gamble of surviving the infection. And you could meliorate that to 99.999% if you're metabolically flexible and vitamin D replete.
And then, really, what are we protecting against with a COVID-19 vaccine? Every bit mentioned, the vaccines aren't even designed to prevent infection, only reduce the severity of symptoms. Meanwhile, they could potentially make you sicker once yous're exposed to the virus. That seems like a lot of take chances for a truly questionable benefit.
To circle back to where we started, participants in current COVID-19 vaccine trials are not being told of this adventure — that by getting the vaccine they may cease up with more astringent COVID-xix once they're infected with the virus.
Lethal Th2 Immunopathology Is Another Potential Take a chance
In closing, consider what this PNAS news feature states about the risk of vaccine-induced allowed enhancement and dysfunction, particularly for the elderly, the very people who would need the protection a vaccine might offer the most:21
"Since the 1960s, tests of vaccine candidates for diseases such equally dengue, respiratory syncytial virus (RSV), and severe astute respiratory syndrome (SARS) take shown a paradoxical phenomenon:
Some animals or people who received the vaccine and were later exposed to the virus developed more severe illness than those who had non been vaccinated. The vaccine-primed immune arrangement, in certain cases, seemed to launch a shoddy response to the natural infection ...
This allowed backfiring, or so-called immune enhancement, may manifest in different ways such as antibody-dependent enhancement (ADE), a procedure in which a virus leverages antibodies to assist infection; or cell-based enhancement, a category that includes allergic inflammation caused by Th2 immunopathology. In some cases, the enhancement processes might overlap ...
Some researchers argue that although ADE has received the most attention to date, it is less likely than the other allowed enhancement pathways to cause a dysregulated response to COVID-nineteen, given what is known about the epidemiology of the virus and its behavior in the human body.
'There is the potential for ADE, but the bigger problem is probably Th2 immunopathology,' says Ralph Baric, an epidemiologist and practiced in coronaviruses ... at the University of N Carolina at Chapel Hill.
In previous studies of SARS, aged mice were establish to accept especially high risks of life-threatening Th2 immunopathology ... in which a faulty T prison cell response triggers allergic inflammation, and poorly functional antibodies that class immune complexes, activating the complement system and potentially dissentious the airways."
Sources and References
- 1 International Journal of Clinical Practice, October 28, 2020 DOI: x.111/ijcp.13795
- 2, 21 PNAS.org April 14, 2020 117 (15) 8218-8221
- iii Viral Immunology 2003;16(one):69-86
- four Science Direct Neutralizing Antibiotic
- five Science Direct Binding Antibody
- 6 Twitter, The Immunologist April 9, 2020
- 7 PLOS Pathogens 2017 Aug; 13(8): e1006565
- 8, 9 Swiss Medical Weekly Apr 16, 2020; 150:w20249
- x Biochemical and Biophysical Research Communications Baronial 22, 2014; 451(two): 208-214
- 11 JCI Insight February 21, 2019 DOI: 10.1172/jci.insight.123158
- 12 PLOS ONE April 2012; vii(4): e35421 (PDF)
- 13 PLOS ONE April 2012; vii(four): e35421 (PDF), page 11
- 14 medRxiv DOI:x.1101/2020.03.30.20047365 (PDF)
- 15 EBioMedicine 2020 May; 55: 102768
- 16 EBioMedicine 2020 May; 55: 102768, Introduction
- 17, 20 Annals of Internal Medicine September two, 2020 DOI: 10.7326/M20-5352
- 18 YouTube, SARS-CoV-2 and the ascension of medical technocracy, Lee Merritt, MD, aprox viii minutes in (Prevarication No. 1: Death Risk)
- nineteen Technical Report June 2020 DOI: 10.13140/RG.2.24350.77125
Live Clean Baby Gentle Moisture Diaper Rash Cream -- 2.6 Oz Reviews
Source: https://www.sott.net/article/445095-How-COVID-19-vaccine-can-destroy-your-immune-system